Strong Acid Strong Base. Weak Acid Strong Base.

Pin On Study Notes In Form Of Ppt Video
Acid Base Titration - Chemistry 1210 Lab report containing an abstract introduction materials procedure.
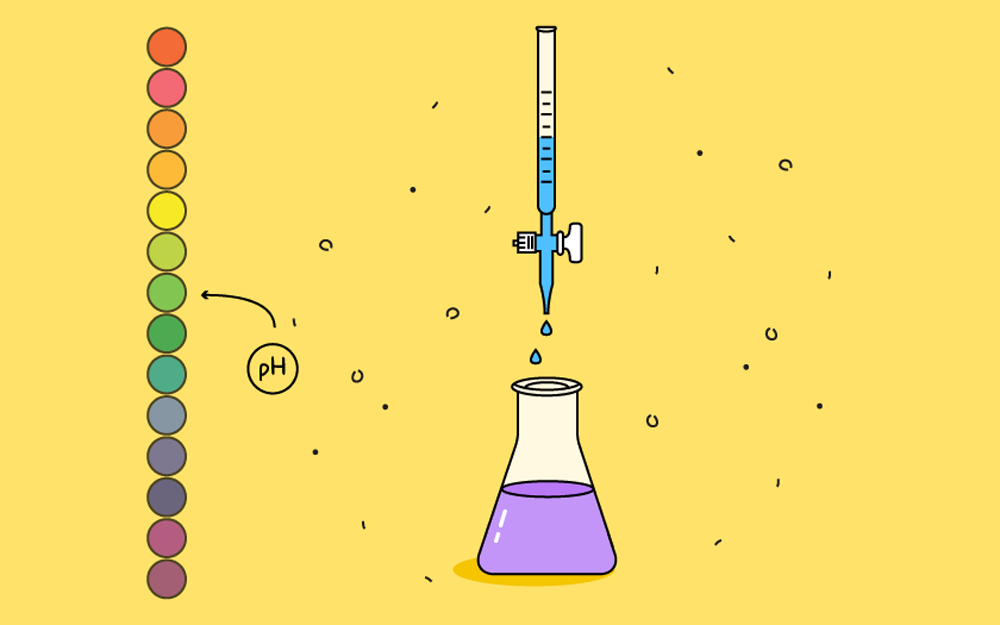
. What should be the molarity of such a sample of the acid if the density of the solution is 1504 gml. This is the base of Chemistry and what must be noted is that a lot of the important objectives - easy marks questions form the base of this chapter appear every year in Class 11 Chemistry Exam. Concise Chemistry Part II - Selina Solutions for Class Chemistry ICSE Chapter 3.
Figure 127 Abasic Sites. Solution 8 a Blue litmus will turn. Acidity is indicated by a pH less than 7 while a pH greater than 7 indicates a base.
You stop to fill your cars gas tank almost making you late for the first day of chemistry class. I All four ii a and d iii b c and d iv only d. Class 11th Chemistry Chapter 1 notes cover.
Iv Only d is correct. Important topics covered in NCERT Solutions for class 7. BANA 2082 - Chapter 22.
Which of these statements are correct. D Change of colour in an acid and a base depends on the type of the indicator. 69 sec 104 sec 7 2 12.
Given that We have 68 nitric acid by mass in aqueous solution which means 68 g nitric acid is present in 100 g of solution. The other sections that could fit within either a general or organicbiological chemistry chapter are sections 56 redox in organic and biochemistry and 75 energy of biochemical reactions. If section 46 were moved to chapter 12 then 56 and 75 would likely need to be moved into an organic or biological chemistry chapter as well.
Get free access to Acids Bases and Salts Class Solutions which includes all the exercises with solved solutions. The electron-withdrawing effect of the substituent makes ionisation easier so successive pK a values decrease in the series 47 28 14 and 07 when 0 1 2 or 3 chlorine atoms are present. As you find a seat in the classroom you read the.
This type of reaction is known as neutralisation. BANA 2082 - Quiz 74 WebAssign. Pingoud A Wilson GG and Wende W.
As we will explore in Chapter 13 methylation of DNA also serves as an important mechanism regulating gene expression. PH is a measurement of the proportion of free hydrogen and hydroxyl ions in water. An AP site apurinicapyrimidinic site also known as an abasic site.
A base reacts with an acid to form a salt and water only. X Y and Z are a general base a Lewis acid and a general acid respectively. Concentrated nitric acid used in laboratory work is 68 nitric acid by mass in aqueous solution.
The differences listed above depicts the clear difference between acids and bases which forms part of the chemistry and discussed among students the world over. A simple example is provided by the effect of replacing the hydrogen atoms in acetic acid by the more electronegative chlorine atom. Another perhaps simpler way to predict the outcome of this reaction is to use the pK a values of the two acids CH 3 CO 2 H 48 and H 2 O 14 clearly acetic acid is a much stronger acid than water and therefore the equilibrium position for this reaction will lie over to the right in favor of the weakest acid and the weakest base.
Apurinic and Apyrimidimic AP sites occur due to unstable hydrolysis. Type II restriction enzymes typically form a homodimer when binding with DNA as shown in the crystal structure of Bgl II in Figure 726B. 2014 Nuc Acids Res 42127489-7527.

Determining Ph Of A Solution Acidic Basic Neutral Solutions Video Lesson Transcript Study Com


0 Comments